The Safety of Controlled Hypotension for Shoulder Arthroscopy in the Beach-Chair Position
Robert Gillespie, MD, Yousef Shishani, MD, Jonathan Streit, MD, J.P. Wanner, BS, Christopher McCrum, BS, Tanvir Syed, MD, Adam Haas, MD, and Reuben Gobezie, MD
Investigation performed at the Case Shoulder and Elbow Service, Case Western Reserve University School of Medicine, Cleveland, Ohio
Background: The safety of controlled hypotension during arthroscopic shoulder procedures with the patient in the beach-chair position is controversial. Current practice for the management of intraoperative blood pressure is derived from expert opinion among anesthesiologists, but there is a paucity of clinical data validating their practice. The purpose of this study was to evaluate the effect of controlled hypotension on cerebral perfusion with use of continuous electroencephalographic monitoring in patients undergoing shoulder arthroscopy in the beach-chair position.
Methods: Fifty-two consecutive patients who had under gone shoulder arthroscopy in the beach-chair position were enrolled prospectively in this study. All patients underwent preoperative blood pressure measurements, assignment of an American Society of Anesthesiologists (ASA) grade, and a preoperative and postoperative neurological and Mini-Mental State Examination (MMSE). The target systolic blood pressure for all patients was 90 to 100 mm Hg during surgery. Continuous intraoperative monitoring was performed with standard ASA monitors and a ten-lead portable electroencephalography monitor. Real-time electroencephalographic monitoring was performed by an attending-level neurophysiologist.
Results: All patients violated at least one recommended limit for blood pressure reduction. The average decrease in systolic blood pressure and mean arterial pressure from baseline was 36% and 42%, respectively. Three patients demonstrated ischemic changes on electroencephalography that resolved with an increase in blood pressure. No adverse neurological sequelae were observed in any patient on the basis of the MMSE.
Conclusions: This study provides the first prospective data on global cerebral perfusion during shoulder arthroscopy in the beach-chair position with use of controlled hypotension. Our study suggests that patients may be able to safely tolerate a reduction in blood pressure greater than current recommendations. In the future, intraoperative cerebral monitoring may play a role in preventing neurological injury in patients undergoing shoulder arthroscopy in the beach-chair position.
View / Download, PDF Version of this Article
The use of controlled hypotension during shoulder arthroscopy with the patient in the beach-chair position is a commonly employed technique with the theoretical benefits of better visualization, decreased blood loss, and shorter operative times1. However, there is controversy about the parameters for the safe administration of controlled hypotension to avoid cerebral ischemia. Current expert opinion in the anesthesia literature advocates an overall systolic blood pressure reduction to no lower than 90 mm Hg and maximum reductions of both systolic blood pressure and mean arterial pressure by no more than 20% of a patient’s baseline values to maintain adequate cerebral perfusion and prevent catastrophic events such as cerebral ischemia 2,3. These recommendations, however, are based on theoretical considerations and case reports rather than data from clinical studies.
The use of electroencephalography is the most sensitive way to monitor for cerebral ischemia in the perioperative and intensive-care-unit setting 4-7. However, to our knowledge, no prospective study has been performed to evaluate the safety of controlled hypotension with electroencephalographic monitoring during shoulder surgery with patients in the beach-chair position.
The purpose of this study was to evaluate the effect of controlled hypotension on cerebral perfusion in patients undergoing shoulder arthroscopy in the beach-chair position with the use of continuous electroencephalographic monitoring. Catastrophic cerebral ischemia in the setting of shoulder surgery is uncommon in both the literature 8 and our anecdotal experience. Thus, we hypothesized that a decrease in blood pressure greater than that recommended by current expert opinion would not be accompanied by evidence of intraoperative cerebral ischemia during, or postoperative neurological sequelae following, shoulder arthroscopy in the beach-chair position.
Disclosure: One or more of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of an aspect of this work. In addition, one or more of the authors, or his or her institution, has had a financial relationship, in the thirty-six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. No author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
Materials and Methods
After approval was granted by our institutional review board, a prospective cohort of fifty-two patients was enrolled into this study. A preoperative power analysis was not performed. Inclusion criteria included all male and female patients at least eighteen years of age who were scheduled to undergo arthroscopic shoulder surgery in the beach-chair position. Exclusion criteria included pregnancy, an age of less than eighteen years, conversion to an open procedure, and refusal to participate in the study. Fourteen patients declined participation in the study. No patient was excluded from the study because of any medical comorbidity (see Appendix), including hypertension, which was defined as a systolic blood pressure of >130 mm Hg or a diastolic blood pressure of >90 mm Hg. All patients had blood pressure measurements obtained from the upper extremity in the clinic preoperatively and again on the day of surgery. In the preoperative waiting area, all patients received a Mini-Mental State Examination (MMSE)9, which is a battery of interview-based questions used to assess multiple areas of cortical function such as attention, expressive language, receptive language, attention, short-term memory, and visuospatial perception. Incorrect responses to select items on the battery correlate with dysfunction of specific cortical regions that may be subject to hypoperfusion injury. This test was administered by anesthesia staff trained and skilled in its interpretation. The MMSE (as opposed to other neurological examinations) was chosen by our multidisciplinary team because it is widely used by the neurological community to discern mental status changes. Patients also had placement of scalp electroencephalography electrodes (Cleveland Biomedical, Cleveland, Ohio) and baseline multichannel electroencephalography performed prior to surgery. All patients received a preoperative American Society of Anesthesiologists (ASA) grade from the attending anesthesiologist. Standard ASA monitors and a ten-lead portable electroencephalography monitor (NeuroMEDIC; NeuroWave, Cleveland, Ohio) were placed on the arm or body outside the operative field prior to induction of anesthesia, with careful attention paid to appropriate blood-pressure-cuff sizing.
Beach-chair positioning at 60° from the horizontal was achieved with the use of a dedicated beach-chair table with a head attachment for all patients. The arthroscopic pump was set at 50 mm Hg, and no epinephrine was introduced into the fluid. Anesthesia was induced with propofol and maintained at 1⁄2 to 1 minimal alveolar concentration of inhaled anesthetic agent via either a laryngeal mask airway or an endotracheal tube. The preoperative interscalene block was successful in all patients. The target systolic blood pressure of 90 to 100 mm Hg was achieved with the combination of beach-chair positioning, the effects of the interscalene block, and titration of an inhaled anesthetic agent. Only one patient required an additional vasoactive agent (labetalol) for further reduction of systolic blood pressure into the target range. Bolus administration of medications that affected electroencephalographic analysis was avoided during the procedure. All instances of electrocautery, hammering, drug administration, and patient manipulation were recorded for critical evaluation of all electroencephalographic data during the post hoc analysis. Blood pressure was recorded at two-minute intervals by an integrated anesthesia-monitoring computer.
Real-time electroencephalographic monitoring was performed by an attending-level neurophysiologist. Cerebral ischemia was defined as a focal or generalized slowing pattern or reduction in overall electroencephalographic amplitude. Decreasing cerebral blood flow is first compensated for by cerebral autoregulation and increased oxygen extraction from hemoglobin. As cerebral blood flow drops further, there is a sequence of electroencephalographic changes that reflect impaired brain cell function, ranging from morphologic changes to burst suppression to an isoelectric electroencephalogram. If these changes persist, brain cell damage or death may occur. If electroencephalographic evidence of cerebral ischemia was detected, the anesthesia team was notified immediately, and the systolic blood pressure was raised until cerebral ischemia was no longer evident. A neurological examination was performed at the first postoperative visit to determine the effects of any intraoperative ischemic events.
All blood pressure measurements were categorized as to whether or not they violated the suggested guidelines of maintaining the systolic blood pressure at >90 mm Hg and maintaining both the mean arterial pressure and the systolic blood pressure within 20% of baseline values3. The baseline systolic blood pressure of each patient, which was the measurement obtained preoperatively in the clinic or in the preadmission testing clinic, was determined from the medical record. All measurements were correlated with any electroencephalographic changes that occurred intraoperatively.
Statistical Analysis
A descriptive analysis of each of several variables (comparing patients with documented intraoperative cerebral ischemia with those who had no electroencephalographic evidence of intraoperative cerebral ischemia) was performed because of the low number of patients with documented intraoperative electroencephalographic changes. The relationship of sex, ASA score, and the presence of medical comorbidities with intraoperative cerebral ischemia was examined with use of the Fisher exact test. The data for ASA score were pooled into two categories: ASA grade 1, or ASA grade 2 or 3. For age, the sample mean and its 95% confidence interval (95% CI) are shown for the two groups, those with and those without an ischemic event. For body mass index (BMI), the median and the interquartile range (IQR) are shown for the two groups as its distribution was too skewed for the sample mean and 95% CI to be appropriate.
Source of Funding
This study was funded in part by the department of orthopaedic surgery at University Hospitals Case Medical Center and NeuroWave Systems, Inc.
Results
Fifty-two patients with a mean age of 52.1 ± 12.7 years, median BMI of 28.9 ± 12.7, mean ASA score of 2.20 ± 0.57 (median = 2), mean preoperative systolic blood pressure of 136.5 ± 16.2 mm Hg, and mean preoperative diastolic blood pressure of 82.1 ± 9.6 mm Hg were enrolled in the study (Table I). The average duration of the surgery was seventy minutes (range, fifty-one to 166 minutes). All fifty-two patients violated at least one of the three blood-pressure-maintenance recommendations, meaning that they had either a drop in systolic blood pressure to < 90 mm Hg (thirty patients), a >20% drop in systolic blood pressure (forty-seven patients), or a >20% drop in mean arterial pressure (fifty-one patients). Forty-six patients violated more than one recommendation. Three of the fifty-two patients sustained events that were associated with electroencephalographic evidence of intraoperative ischemia (Table II). There were no adverse neurological sequelae in any patient based on the postoperative MMSE. Results, including the duration of violations and associated ischemia, are reported in Table III.
Overall, the percentage decrease in systolic blood pressure and mean arterial pressure for the fifty-two patients was a mean of 36% and 42%, respectively (95% CI: 32.40% to 38.72% and 95% CI: 38.20% to 45.65%, respectively). In the patients who did not sustain an ischemic event, the mean percentage drop in systolic blood pressure and mean arterial pressure was 35% and 41.5%, respectively (95% CI: 31.64% to 38.14% and 95% CI: 37.61% to 45.40%, respectively). In the patients who did demonstrate evidence of an ischemic event on electroencephalography, the mean percentage drop in systolic blood pressure and mean arterial pressure were 47% and 49%, respectively (95% CI: 35.15% to 57.91% and 95% CI: 26.84% to 70.69% , respectively) (Figs. 1 and 2). All patients with documented ischemia experienced systolic blood pressure of <90 mm Hg, whereas twenty-seven patients tolerated a systolic blood pressure of <90 mm Hg without evidence of ischemia.
Because of the relative rarity of ischemia, meaningful statistical analysis of the effect of a number of variables on the occurrence of ischemia was not possible.
The predictive quality of the recommended blood pressure parameters was analyzed. There was a higher correlation of ischemic events with violations due to a failure to maintain systolic blood pressure above 90 mm Hg, despite not reaching significance (p = 0.184) (Table IV).
Table I: Demographics of Patient Cohort
| Ischemia* (N = 3) | No Ischemia* (N = 49) | All Patients (N = 52) | |
| Sex (no. [%]) | |||
| Female | 2 (15.4%) | 11 (84.6%) | |
| Male | 1 (2.6%) | 38 (97.4%) | |
| Smoker (no. [%]) | |||
| Yes | 1 (12.5%) | 7 (87.5%) | |
| No | 2 (4.5%) | 42 (95.5%) | |
| Hypertension (no. [%]) | |||
| Yes | 1 (3.4%) | 28 (96.6%) | |
| No | 2 (8.7%) | 21 (91.3%) | |
| Diabetes (no. [%]) | |||
| Type II | 2 (50%) | 2 (50%) | |
| None | 1 (2.1%) | 47 (97.9%) | |
| BMI (no. [%]) | |||
| <25 kg/m2 | 2 (22.2%) | 7 (77.8%) | |
| 25-30 kg/m2 | 0 (0%) | 24 (100%) | |
| >30 kg/m2 | 1 (5.3%) | 18 (94.7%) | |
| Mean age (range) (yr) | 52 (18-77) | ||
| Comorbidities (no. [%]) | |||
| Yes | 41 (78.8%) | ||
| No | 11 (21.2%) |
* The values are expressed as the percentage of the patients within each category (e.g., female or BMI of >25 kg/m2) who did or did not have ischemia.
Table II: Details of Patients Experiencing Ischemia
| Case | Age (yr) | Procedure | Surgery Duration (min) | Ischemia Time (min) |
| 1 | 49 | Bankart repair | 51 | 18 |
| 2 | 58 | Massive rotator cuff repair, subacromial decompression, biceps tenodesis | 87 | 13 |
| 3 | 45 | Subacromial decompression, capsular release, biceps tenodesis |
84 | 6 |
| Average for all patients in study | 52 | 70 | NA |
Table III: Duration and Nature of Blood Pressure Guideline Violations*
| Systolic Blood Pressure >90 mm Hg | <20% Reduction in Mean Arterial Pressure | <20% Reduction in Systolic Blood Pressure | Total No. of Violations† | Blood Pressure Violations Associated with Ischemia‡ | Percent of Violations Associated with Ischemia | Percent of Violations Not Associated with Ischemia | |
| Total | 157 | 943 | 740 | 1777 | 18 | 1.01% | 98.99% |
* Blood pressure readings were performed at two to five-minute intervals. The values are given as the number of instances unless otherwise indicated. † An instance in which more than one guideline was violated was considered to be one violation. ‡ The total number of readings in which simultaneous electroencephalographic evidence of ischemia was noted.
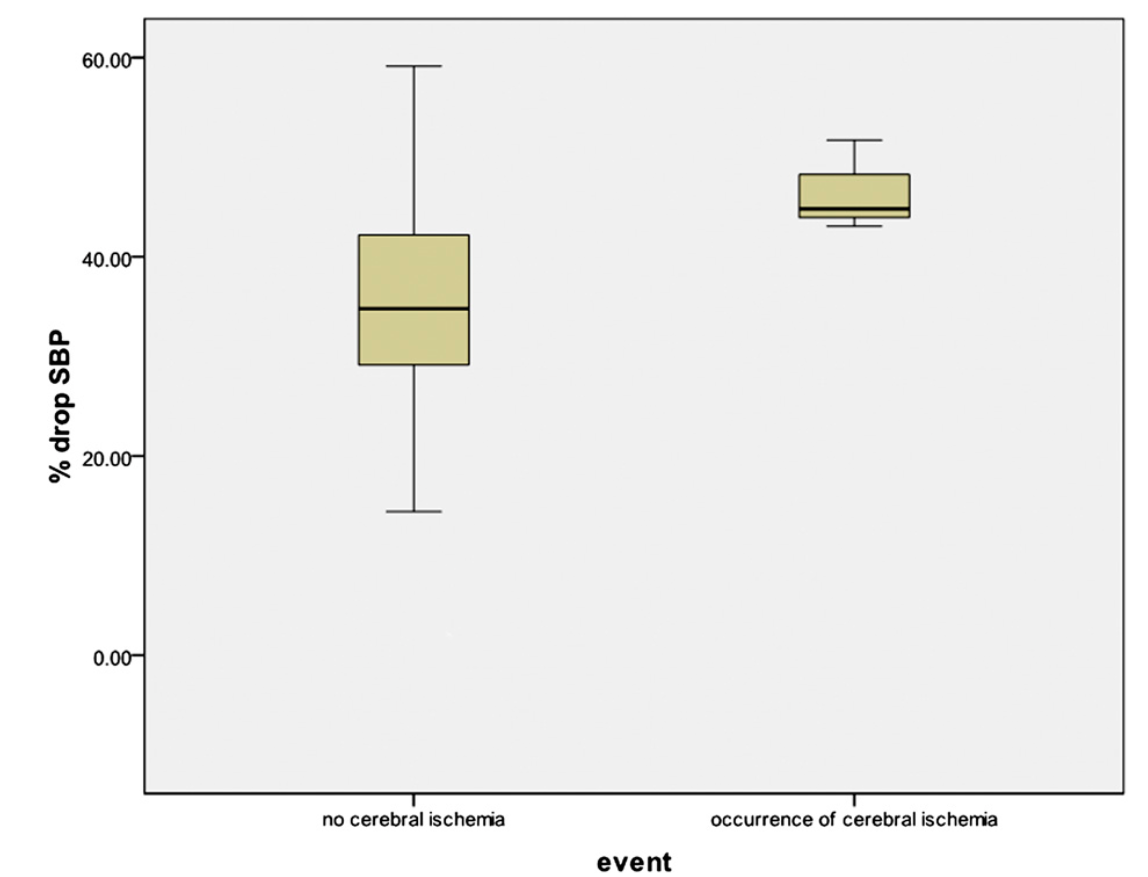
Fig. 1
Cerebral ischemia as a function of the percent drop in systolic blood pressure (SBP).
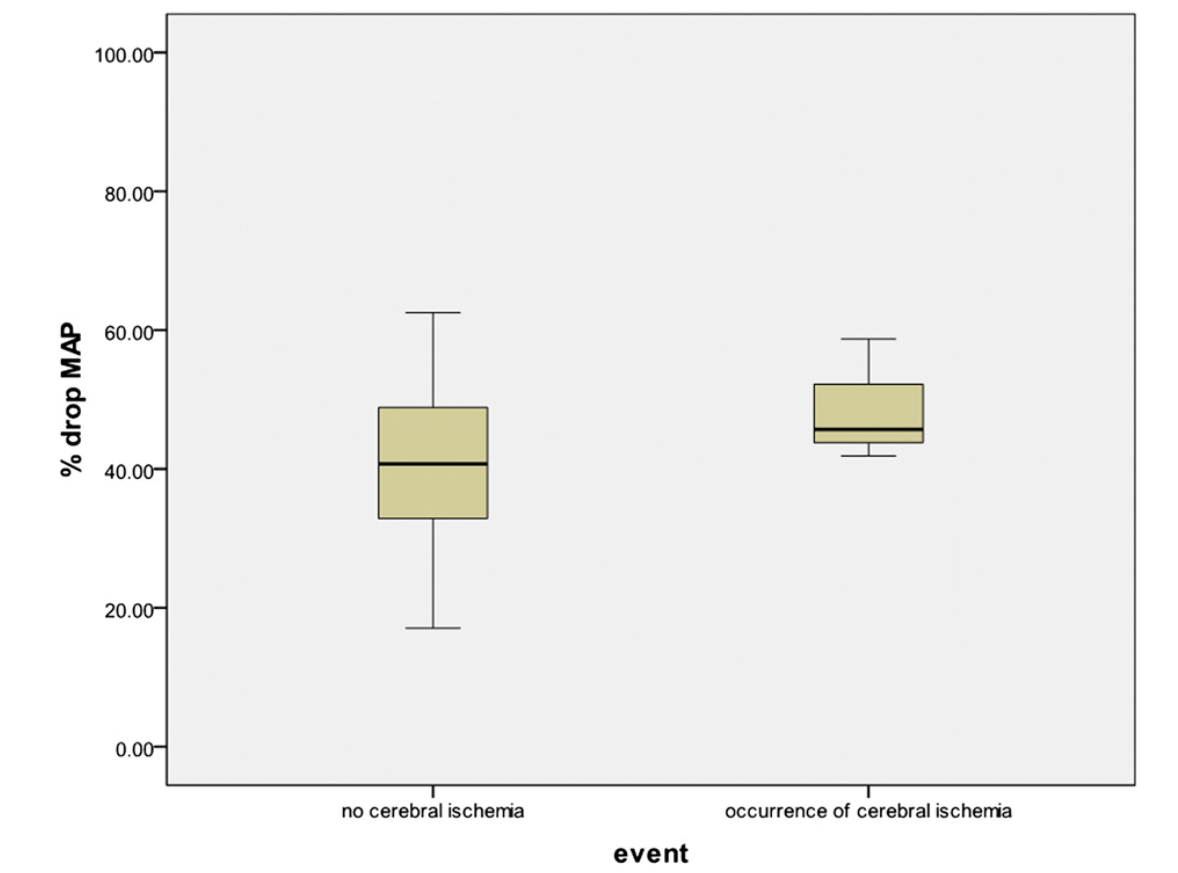 Fig. 2
Fig. 2
Cerebral ischemia as a function of the percent drop in mean arterial pressure (MAP).
Table IV: Ischemia as a Function of Systolic Blood Pressure (P = 0.184)*
| Violation of Systolic Blood Pressure of >90 mm Hg | |||
| Event | No | Yes | Total |
| No cerebral ischemia | 22 | 27 | 49 |
| Occurrence of cerebral ischemi | 0 | 3 | 3 |
| Total | 22 | 30 | 52 |
* The values are given as the number of patients.
Discussion
The beach-chair position is used commonly in arthroscopic shoulder procedures and has been implicated as an independent risk factor for cerebral ischemia during surgery2,4,5,10. Expert-opinion recommendations regarding blood-pressurereduction limits have been made by prominent anesthesiologists to minimize the risk of cerebrovascular insults to the patient3,11,12, but there is little clinical evidence other than case reports and theoretical considerations to support these guidelines. To our knowledge, ours is the first prospective study on the effects of controlled hypotension on the risk of cerebral ischemia during shoulder arthroscopy with the patient in the beach-chair position with use of continuous electroencephalographic monitoring. Our data suggest that patients may be able to tolerate a greater drop in blood pressure than indicated by current recommendations, as a mean short-term maximum reduction of 35% in systolic blood pressure and a 42% reduction in the mean arterial pressure resulted in no electroencephalographic changes in our patient cohort. Patients who demonstrated evidence of cerebral ischemia on intraoperative electroencephalographic monitoring showed a trend toward greater reductions in systolic blood pressure and mean arterial pressure compared with the patients who showed no evidence of intraoperative ischemia; these findings support the idea that a blood pressure threshold exists. All ischemic events resolved with blood pressure elevation, and none resulted in any clinically identifiable neurological sequelae based on MMSE.
There is a known change in blood pressure that occurs in patients placed in the beach-chair position. However, the effect of the sitting position on cerebral perfusion is highly controversial and has been the subject of much public debate3,11-13. It is believed that cerebral perfusion pressure decreases proportional to the elevation of the head relative to the heart in patients sitting upright. In a recent study, Murphy et al.13, utilizing cerebral oximetry, found that approximately 80% of patients experienced a drop in cerebral oxygenation when moving from the supine to the sitting position, even when systolic blood pressure was kept within 20% of baseline. However, the authors of that study acknowledged that many factors can affect cerebral oximetry, including the relative composition of arterial and venous blood being assessed in each position. Our findings based on electroencephalographic monitoring suggest that the incidence of cerebral oximetry changes may not correlate with the occurrence of cerebral ischemia, given the high incidence of cerebral oximetry changes in the study by Murphy et al. but the comparatively low incidence of reversible cerebral ischemia demonstrated by electroencephalographic monitoring in our study. In theory, the combination of controlled hypotension and the sitting position can lead to undetected cerebral ischemia in an anesthetized patient undergoing arthroscopic shoulder surgery in the beach-chair position14-16. More work needs to be done as this theoretical risk of ischemia has not been convincingly validated in clinical studies and the present study was limited in that only three occurrences of reversible ischemia were detected by electroencephalography.
In 2005, Pohl and Cullen reported on a case series of four patients who demonstrated various degrees of neurological compromise following shoulder surgery in the beach-chair position2. They noted the dangers of deliberate hypotension in the upright position and suggested a multitude of contributory factors ranging from improper placement of the blood pressure cuff to incorrect head positioning. There were numerous limitations to the study, but it clearly highlighted the need for clarification of the guidelines surrounding the use of deliberate hypotension in the beach-chair position.
Papadonikolakis et al.17 recently discussed the interpretation of blood pressure during induced hypotension to avoid iatrogenic cerebral hypoperfusion, but no definitive guidelines could be determined. In our study, no patient whose systolic blood pressure remained above 90 mm Hg sustained an ischemic event. Of note, the target systolic blood pressure for this study was 90 to 100 mm Hg, and instances of systolic blood pressure of >90 mm Hg would have been corrected irrespective of electroencephalographic changes. However, blood pressure titration is inexact and instances of “overshoot” in blood pressure reduction should be expected when controlled hypotension is attempted. This study also suggests that patients who experienced an ischemic event were more likely to have had greater percentage decreases in their systolic blood pressure and mean arterial pressure than patients without an ischemic event. The findings of our study were consistent with the established blood pressure guidelines for eliminating ischemic events intraoperatively. However, the findings suggest that these guidelines may be too stringent. Because of the rarity of catastrophic events related to intraoperative cerebral ischemia, more work needs to be done to elucidate the role of blood pressure monitoring in the prediction of cerebral ischemia in patients undergoing shoulder surgery in the beach-chair position.
Electroencephalography is one of the most commonly used modalities for intraoperative neuromonitoring4-7,14. Various forms of electroencephalography have been studied for the detection of cerebral ischemia, and they have shown good sensitivity and reproducibility5,6. In our study, a trained neurophysiologist performed real-time and post hoc interpretation of the electroencephalographic data to minimize false-negative rates for ischemia detection and to immediately implement interventions to correct cerebral ischemia when it occurred. Other currently available monitors available for aiding in the detection of cerebral ischemia have substantial limitations. Although use of indwelling arterial catheters is an excellent way to monitor peripheral blood pressure and, in the setting of normal intracranial pressure, the results can correlate with those of cerebral perfusion, they are more invasive than external monitors. Regional cerebral oximetry has also been described for monitoring of cerebral ischemia, and it is an attractive option secondary to its relatively lower cost and simplicity of use. However, in a recent review of the literature, Smith and Elwell found this technology to be unproven for detecting cerebral ischemia18. In addition, Friedell et al. showed that regional cerebral oximetry did not correlate with, or add new information to, electroencephalography and somatosensory evoked potentials (SSEPs) during carotid endarterectomy14. Others have examined transcranial Doppler ultrasound in an attempt to find more accurate modalities for detecting cerebral ischemia, but these studies have been limited because of technical difficulty of the monitoring19 or because it could not be performed on 10% to 15% of the population due to the absence of a temporal bone window20. In addition, transcranial Doppler ultrasound only monitors flow within specific arteries and provides no information regarding cerebral ischemia or perfusion19,20. SSEPs only give information about one part of the brain and are not a measure of global ischemia, and are therefore of limited use during shoulder surgery in the beach-chair position14. It is clear from these studies that electroencephalography remains the gold standard for monitoring cerebral ischemia when used by those highly skilled in the interpretation of its results. Although continuous electroencephalographic monitoring is time-consuming and requires highly trained analysts, it remains the most reliable monitor of cerebral ischemia in anesthetized patients undergoing controlled hypotension during surgery in the beach-chair position.
There are several limitations to this study. One is the relatively short follow-up in this cohort for the detection of neurological sequelae. However, there is no reason to believe that these sequelae would appear after the initial postoperative period. Additionally, the small number of patients who experienced electroencephalographic changes in this prospective cohort limited the power and statistical methods as well as the conclusions that could be drawn from our study population. Another limitation is the lack of a control group such as a cohort of patients who received an operation in a sitting position with maintenance of normal blood pressure or in the supine position with controlled hypotension. In a recent article by Friedman et al.8, the relationship between patient positioning and the incidence of cerebrovascular events was estimated after a survey was sent to orthopaedic shoulder surgeons who self-reported their complications. Approximately 200,000 shoulder surgical procedures had been performed by the surgeons who returned the survey. Although Friedman et al. found no significant difference in the incidence of events between the lateral decubitus position and the beach-chair position, no cerebrovascular events occurred in patients undergoing a procedure in the lateral position while eight events occurred in those in the beach-chair position. The overall risk of a cerebrovascular event was 0.003%, highlighting the difficulty in studying controlled hypotension with a cerebrovascular event as the end point. Thus, the use of a system such as electroencephalography, which can detect earlier cerebral ischemic changes, for intraoperative monitoring may be warranted in addition to setting clear blood pressure guidelines to prevent catastrophic cerebrovascular events.
In conclusion, the results of this study suggest that patients may be able to tolerate greater reductions in blood pressure during arthroscopic shoulder surgery performed in the beach-chair position than are currently allowed by guidelines based on expert opinion3,11. This finding is important because the use of controlled hypotension leads to better visualization, to minimize surgical time, and maximizes the potential for successful surgical repair. According to the findings of previous studies and case reports, however, controlled hypotension in the beach-chair position may increase the risk of catastrophic neurological outcomes in some patients. Further research is required to understand which patients are at highest risk for cerebral ischemia during shoulder surgery in the beach-chair position and the role of neuromonitoring during these procedures.
Appendix
A table showing comorbidity in each group is available with the online version of this article as a data supplement at jbjs.org.
NOTE: The authors acknowledge Troy Mounts, MD, for data collection.
Robert Gillespie, M.D., Yousef Shishani, M.D., Jonathan Streit, M.D., J.P. Wanner, B.S., Christopher McCrum, B.S., Tanvir Syed, M.D., Adam Haas, M.D.,
Reuben Gobezie, M.D., The Case Shoulder and Elbow Service (R. Gillespie, Y.S., J.S., J.P.W., C.M., and R. Gobezie), Department of Neurology (T.S.), and Department of Anesthesia (A.H.),
11100 Euclid Avenue, HH5043,
Cleveland, OH 44106.
E-mail address for R. Gobezie: clevelandshoulder [at] gmail.com
References
- Paul JE, Ling E, Lalonde C, Thabane L. Deliberate hypotension in orthopedic surgery reduces blood loss and transfusion requirements: a meta-analysis of randomized controlled trials. Can J Anaesth. 2007;54:799-810.
- Pohl A, Cullen DJ. Cerebral ischemia during shoulder surgery in the upright position: a case series. J Clin Anesth. 2005;17:463-9.
- Lanier W. Cerebral perfusion: err on the side of caution. Anesthesia Patient Safety Foundation Newsletter. 2009 Spring;24:1-24.
- Tan TW, Garcia-Toca M, Marcaccio EJ Jr, Carney WI Jr, Machan JT, Slaiby JM. Predictors of shunt during carotid endarterectomy with routine electroencephalography monitoring. J Vasc Surg. 2009;49:1374-8.
- Kurtz P, Hanafy KA, Claassen J. Continuous EEG monitoring: is it ready for prime time? Curr Opin Crit Care. 2009;15:99-109.
- Isley MR, Edmonds HL Jr, Stecker M; American Society of Neurophysiological Monitoring. Guidelines for intraoperative neuromonitoring using raw (analog or digital waveforms) and quantitative electroencephalography: a position statement by the American Society of Neurophysiological Monitoring. J Clin Monit Comput. 2009; 23:369-90. Epub 2009 Sep 16.
- Friedman D, Claassen J, Hirsch LJ. Continuous electroencephalogram monitoring in the intensive care unit. Anesth Analg. 2009;109:506-23.
- Friedman DJ, Parnes NZ, Zimmer Z, Higgins LD, Warner JJ. Prevalence of cerebrovascular events during shoulder surgery and association with patient position. Orthopedics. 2009;32. pii:orthosupersite.com/view.asp?rID=38058.
- Folstein MF, Folstein SE, McHugh PR. ‘‘Mini-mental state’’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189-98.
- Mazzon D, Danelli G, Poole D, Marchini C, Bianchin C. Beach chair position, general anesthesia and deliberated hypotension during shoulder surgery: a dangerous combination! Minerva Anestesiol. 2009;75:281-2.
- Munis JR. The problem of posture, pressure, and perfusion. Anesthesia Patient Safety Patient Safety Foundation Newsletter. 2008;22:82-3.
- Hicks JW, Munis JR. The siphon controversy counterpoint: the brain need not be ‘‘baffling’’. Am J Physiol Regul Integr Comp Physiol. 2005;289:R629-32.
- Murphy GS, Szokol JW, Marymont JH, Greenberg SB, Avram MJ, Vender JS, Vaughn J, Nisman M. Cerebral oxygen desaturation events assessed by nearinfrared spectroscopy during shoulder arthroscopy in the beach chair and lateral decubitus positions. Anesth Analg. 2010;111:496-505. Epub 2010 May 27.
- Friedell ML, Clark JM, Graham DA, Isley MR, Zhang XF. Cerebral oximetry does not correlate with electroencephalography and somatosensory evoked potentials in determining the need for shunting during carotid endarterectomy. J Vasc Surg. 2008;48:601-6. Epub 2008 Jul 18.
- Drummond JC. The lower limit of autoregulation: time to revise our thinking? Anesthesiology. 1997;86:1431-3.
- Kirby RR, Cullen DJ. Complications in the beach chair position. In: Lobato EB, Gravenstein N, Kirby RR, editors. Complications in anesthesiology. Philadelphia: Lippincott Williams and Wilkins; 2008: p 844-53.
- Papadonikolakis A, Wiesler ER, Olympio MA, Poehling GG. Avoiding catastrophic complications of stroke and death related to shoulder surgery in the sitting position. Arthroscopy. 2008;24:481-2.
- Smith M, Elwell C. Near-infrared spectroscopy: shedding light on the injured brain. Anesth Analg. 2009;108:1055-7.
- Moritz S, Kasprzak P, Arlt M, Taeger K, Metz C. Accuracy of cerebral monitoring in detecting cerebral ischemia during carotid endarterectomy: a comparison of transcranial Doppler sonography, near-infrared spectroscopy, stump pressure, and somatosensory evoked potentials. Anesthesiology. 2007;107:563-9.
- Pennekamp CW, Bots ML, Kappelle LJ, Moll FL, de Borst GJ. The value of nearinfrared spectroscopy measured cerebral oximetry during carotid endarterectomy in perioperative stroke prevention. A review. Eur J Vasc Endovasc Surg. 2009;38: 539-45. Epub 2009 Aug 7.


