Biologic and Synthetic Grafts in the Reconstruction of Large to Massive Rotator Cuff Tears
Robert J. Gillespie, M.D., Derrick M. Knapik, M.D., Ozan Akkus, PhD
Abstract
Rotator cuff injuries are common in both young and elderly patients. Despite improvements in instrumentation and surgical techniques, the failure rates following tendon reconstruction remain unacceptably high. To improve outcomes, graft patches have been developed to provide mechanical strength and to furnish a scaffold for biologic growth across the delicate tendon-bone junction. Although no patch effectively re-creates the structured, highly organized system of prenatal tendon development, augmenting rotator cuff repair may help restore native tendon-to-bone attachment while reproducing the mechanical and biologic properties of native tendon. An understanding of biologically and synthetically derived grafts, along with knowledge of the preliminary data available regarding their combined use with growth factors and stem cells, is needed to improve management and treatment outcomes. The current literature has not been consistent in showing patch augmentation to be beneficial over traditional repair, but novel scaffolding materials may help facilitate rotator cuff tendon repair that is histologically and biomechanically comparable to native tendon.
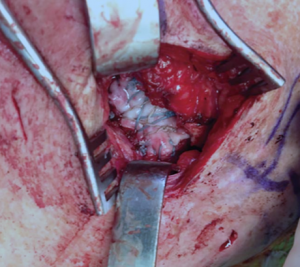
Intraoperative photograph showing the implantation of an extracellular matrix graft using an open approach to augment the repair of a massive rotator cuff tear not amendable by primary repair.
Rotator cuff disease represents the most common disability afflicting the upper extremity and is a serious cause of disability in both young and elderly patients.1 Most rotator cuff tears occur secondary to chronic degeneration of the intrinsic rotator cuff tendons, compromising the quality and integrity of the cuff and leading to progressive tearing and failure.2 Despite advances in instrumentation and surgical techniques, including novel suture materials, modified stitch designs, and inventive fixation devices, the reconstruction of massive rotator cuff tears—those measuring .5 cm or involving two torn tendons—remains a challenge for orthopaedic surgeons.
Repair failure and re-tears following the reconstruction of large to massive rotator cuff defects occur in 20% to 90% of patients.3 The high failure rate largely is attributed to the complex anatomic structure of the rotator cuff and the high functional demands placed on the tendons during normal daily function.1 In particular, tendon-to-bone healing at the enthesis following injury fails to fully regenerate the native histologic composition and the mechanical strength of normal tendon. Subsequently, repaired tendons lack elasticity, resulting in stiffer, mechanically inferior scar tissue that is prone to failure.4
Because of the high rates of failure following conventional rotator cuff repair in large tears, investigation into new strategies is warranted to reduce the re-tear rates and improve longterm clinical and functional outcomes. Advances in tissue engineering seek to improve native tendon healing using scaffolds, growth factors, and stem cells, which augment repair by promoting biologic healing and cellular ingrowth into tissue while providing adequate mechanical reinforcement. These advances aim to restore the physiologic mechanical strength of the tendon while aiding biologic regeneration at the critical tendon-bone interface.5
Here, we focus on the current and novel tissue-engineering strategies used to augment rotator cuff repair of large to massive tears in humans. We provide an overview of the available biologic and synthetic augmentation patches and of prospective growth factors and stem cell–infused devices for the treatment of rotator cuff tears.
Extracellular Matrix Patch Scaffolds
Acellular, extracellular matrix grafts serve as a tissue bridge between the torn tendon and the bone at the enthesis, providing a scaffold for cellular growth and collagen formation during the reconstruction of large to massive rotator cuff lesions.6 Graft patches provide mechanical strength and supply the biologic properties essential to the healing of the torn tissue. Compared with native tendons, however, extracellular matrix scaffolds possess inferior mechanical properties and cannot fully re-create the highly structured and organized system of tendon remodeling seen in native tendon development.7 Scaffolds can be implanted using an open or an arthroscopic technique, depending on surgeon preference; both techniques allow visualization of the repaired tendon and ensure the re-creation of the native enthesis at the humerus (Figure 1).
Scaffolds are developed from biologic tissues or are manufactured using synthetic material. Biologic scaffolds are classified according to the type of cells used, commonly fascia lata, dermal, or small intestine submucosa, as well as their tissue source, including xenografts, human autograft, or human allograft. Animal studies have demonstrated mechanical and biologic benefit using biologic grafts, but human studies have demonstrated less promising results.
Xenograft
The Restore Orthobiologic Implant (DePuy Synthes) is an acellular, non– cross-linked collagen-based patch made from porcine small intestine mucosa. Studies in human subjects have demonstrated little to no clinical or functional improvement when using this patch compared with conventional repair. Studies have cited high rates of incomplete healing,8 inferior range of motion, postoperative inflammation, and re-tear and complication rates similar to those of the control groups.9 Some authors have pointed to the high levels of DNA present within the patch’s matrix to account for the patch’s high rates of inflammation and inferior outcomes.7,10 As a result of its immunogenicity and poor outcomes, the use of the Restore patch in cuff repair is discouraged.10
The Zimmer Collagen Repair Patch (ZCR, also known as Permacol) is an acellular sheet of cross-linked porcine dermis. Human studies have reported variable outcomes using ZCR. Favorable studies cite improvement in outcomes at short-term follow-up according to the University of California, Los Angeles Shoulder score (P = 0.042) and the American Shoulder and Elbow Surgeons (ASES) Shoulder score (P = 0.043). At long-term follow-up, outcomes include improved Constant scores (P = 0.0003),11,12 significant reductions in postoperative pain as demonstrated by improved visual analog scale pain scores (P = 0.041,11 P , 0.0512), and significant long-term increases in strength (P , 0.05) and range of motion (P , 0.05). High satisfaction rates among patients were observed, as well.11,12 Imaging at short-term and long-term evaluations demonstrated intact grafts in approximately 80% of patients.11,12 However, both studies11,12 failed to incorporate a control group to assess outcomes in comparison with conventional rotator cuff repair. The study by Gilbert et al10 supported the use of ZCR and reported no measurable quantities of DNA or complications relating to inflammation in patients. Conflicting studies on the use of ZCR cite poor clinical outcomes, with no marked improvement in shoulder scores in patients treated for large or massive cuff repairs and no improvement in re-tear rates.13,14 In a small series, Soler et al14 reported inflammatory reactions in 4 of 4 patients as well as evidence of graft disintegration, tissue necrosis, and re-tears; however, the patients were aged 71 to 82 years, decreasing the likelihood of successful graft integration and survival because of suboptimal tissue quality in the elderly. Therefore, no consensus exists regarding the reliability of the ZCR patch.
CuffPatch (Arthrotek) is an acellular, cross-linked sheet made from porcine small intestine submucosa. The data on the use of CuffPatch in humans are limited. Substantial inflammation in some hosts has been reported,2 but other patients have demonstrated negligible amounts of DNA.7 Thus, the use of CuffPatch is neither recommended nor contraindicated based on the available research data.
The Conexa Reconstructive Tissue Matrix (Tornier) is made from porcine dermis. Only a single human case series examining 27 shoulders in 26 patients with massive or two-tendon rotator cuff tears has investigated the use of the Conexa patch. At an average of 32 months, significant improvement was observed in ASES (P = 0.0007) and Medical Outcome 12-Item Short-Form Health Survey (P = 0.044) clinical outcome scores.15 Improvement in strength (P = 0.001), forward flexion (P = 0.024), and abduction (P = 0.001), as well as a significant reduction in pain levels (P = 0.002), was also noted. Of 27 patients, 16 (73%) had intact graft reconstructions visible on ultrasonography at final follow-up, with only a single patient (5%) exhibiting a complete re-tear. Additional human studies are required before the viability and potential of the Conexa patch in rotator cuff repair can be predicted reliably.
Only a single retrospective study has examined use of the highly porous, highly aligned reconstituted bovine collagen implant distributed by Rotation Medical. Arnoczky et al16 noted over 350 procedures being performed using this implant, with no adverse events reported up to 20 months following index surgery. A total of five patients with full-thickness rotator cuff tears treated with patch augmentation underwent second-look arthroscopy with tissue biopsy because of pain (n = 1), trauma (n = 3), or staged hemiarthroplasty (n = 1) at an average of 3 months from surgery (range, 5 weeks to 6 months). Histologic analysis revealed host cell incorporation within the implant, with evidence of cell alignment along the linear orientation of the implant in 5and 8-week samples. Three-month samples (n = 2) demonstrated increased collagen formation and organization over the implant, while the 6-month sample revealed the presence of highly oriented collagen fibers, indicative of functional host tissue loading. No evidence of any inflammatory or foreign body reaction was present in any of the samples. Additional prospective studies evaluating functional outcome scores and complications in humans against control subjects are warranted to determine true graft viability.
TissueMend Soft Tissue Repair Matrix (TEI Biosciences) is an acellular, nondenatured collagen membrane derived from fetal bovine dermis lacking artificial crosslinkage. No human studies have examined TissueMend; however, Derwin et al17 reported substantially higher levels of DNA within the TissueMend matrix than in other xenografts patches.
Allografts
The GraftJacket Matrix (Wright Medical Technology) is an acellular, non–cross-linked dermal graft created using the epidermal and dermal layers of human skin. In a recent randomized controlled trial of 22 patients undergoing treatment with augmentation for large two-tendon tears .3 cm, Barber et al18 found significant improvement in ASES (P = 0.035) and Constant (P = 0.008) scores in patients treated with the graft. Of subjects receiving the patch, 85% demonstrated intact cuff repair on MRI compared with 40% of control subjects at a mean follow-up of 14.5 months. GraftJacket patches are marketed as an acellular material, but researchers have detected DNA on the grafts,7,10 which could produce inflammation, causing subsequent pain and edema at the repair site and increasing the risk of potential degradation.19 GraftJacket has been shown to be well tolerated and durable, however. Intact grafts have been noted in 85% to 93% of patients at 14.5 to 36 months,18,20 with few reported complications.21,22
AlloPatch Pliable (Musculoskeletal Tissue Foundation) is an allogeneic matrix made from harvested human fascia lata. In a recent retrospective review of 14 patients with large to massive tears and a previously failed cuff repair, Agrawal23 found that 12 patients (85.7%) had intact repairs visible on MRI at an average of 16.8 months. Significant improvements in the Constant score (P = 0.009), the pain score (P = 0.008), scapular plane abduction (P = 0.010), and strength (P = 0.006) were reported, as well. The authors concluded that the AlloPatch is a safe and effective graft for the repair of massive to large revision cuff tears. To date, however, no studies have evaluated the longterm structural or clinical performance of AlloPatch in humans.
Synthetic Grafts
Synthetic grafts are made from various chemical compounds and consist of polymers made from polyester, polypropylene, polyacrylamide, Dacron, carbon, silicon, or nylon. Studies have shown synthetic grafts to be mechanically stronger than biologic grafts, to be consistent in quality, and to pose minimal risk of disease transmission.24 Biocompatibility concerns remain, however, because reactions secondary to the placement of synthetic foreign bodies increase the risks of infection, instability, synovitis, osteolysis, and osteoarthritis.24 Few human studies have investigated the efficacy of synthetic grafts in repairing large to massive rotator cuff tears; most studies have used animal models.
A recent study found that 15 of 18 patients with massive rotator cuff tears reinforced with the poly(L-lactide) patch, X-Repair (Synthasome), had intact repairs by 12 months noted on ultrasonography, and ASES scores improved from 25 to 71 by 12 months (P = 0.03). No statistically significant difference in ASES scores was observed between 12 and 42 months, however (P . 0.1).25 Although the literature is limited on the biocompatibility of X-Repair with host tissues, Derwin et al17 reported evidence in a canine model of fibrous tissue in-growth within grafts without evidence of neutrophils or lymphocytes following rotator cuff augmentation. Therefore, additional human data are necessary to determine whether the strength and viability of the X-Repair graft can withstand long-term implantation.
A 3-year cohort study of the polypropylene patch compared the synthetic graft versus traditional open repair without augmentation. Repairs were augmented with the Tutopatch collagen bovine pericardium-derived patch (Tutogen Medical GmbH).26 Patients treated with the polypropylene patch had significantly higher University of California, Los Angeles Shoulder scores (P , 0.001), mean elevation (P , 0.001), and abduction strength (P , 0.001) at 36 months than did those treated with repair only or with a Tutopatch. The polypropylene patch also demonstrated superior durability, with retear rates of only 17%, compared with rates of 41% in the control group and 51% in the Tutopatch group, demonstrating the polypropylene patch to be a viable augmentation graft.
The Biomerix RCR Patch (Biomerix) is made from a polycarbonate polyurethane-urea nonbiodegradable polymer. A clinical series that examined 10 patients with full-thickness supraspinatus or infraspinatus tendon tears demonstrated significant improvement in clinical outcomes as measured by the Simple Shoulder Test and ASES scores, pain, and range of motion at 6 and 12 months (P , 0.05 and P , 0.01, respectively).27 Furthermore, only a 10% failure rate (visible on ultrasonography and MRI) was noted at 12 months. The study was limited by the lack of a control group, however, and included primarily small to medium (mean size, 20 mm) tendon tears, necessitating additional human studies before a definitive conclusion regarding its use can be reached.
Only limited clinical data are available on the use of other available synthetic patches in humans.28,29 Nada et al28 evaluated the Dacron polyester ligament graft in 21 patients over a 3-year study course and found that 95% of patients reported considerable pain relief, with statistically significant increases in Constant scores (P , 0.001). The Gore-Tex Soft Tissue Patch, a polyethylene terephthalate synthetic patch, has demonstrated excellent biocompatibility in massive cuff tears in humans, but no clinical outcome measures have supported its compatibility.29 To date, the polyurethane urea patch Sports Mesh (Zimmer Biomet) has not been evaluated in human studies.
Combinations
Recent studies have evaluated augmentation of scaffolds with growth factors and mesenchymal stem cells to manage large to massive rotator cuff defects. Although these novel combinations are promising, no data for them are available in humans; studies have been restricted to only biomechanical and animal models.
In an ovine model, the addition of recombinant human plateletderived growth factor BB to a collagen matrix scaffold demonstrated superior histologic properties and structural graft strength.30 Zhao et al31 combined basic fibroblast growth factor into electrospun poly(lactide-co-glycolide) (PLGA) fibrous scaffolding in rats and found excellent biocompatibility and biodegradability, with considerable improvement in collagen organization at 2, 4, and 8 weeks compared with control specimens and PLGA repair-only specimens. Yokoya et al32 combined a polyglycolic acid (PGA) sheet seeded with autologous cultured bone marrow mesenchymal stem cells (bmMSCs) in a rabbit model and demonstrated a higher ratio of collagen type I to III in the PGAbmMSC group than in the PGAonly group, with greater tendon maturing values than in the PGA and control groups at 8 and 16 weeks. The study also found superior biomechanical results in the PGA-bmMSC groups, in whom regenerated tendons possessed markedly better tensile strength than those in the PGA and control groups at 16 weeks. Therefore, advancements in tissue engineering and cellular development show potential in augmenting the surgical repair of rotator cuff tears, but human studies are necessary to assess translational success.
Aurora et al33 designed a novel reinforced fascia patch using poly-Llactic acid (PLLA) or PLLA/PGA polymer braids. Testing in a rat model demonstrated a maximum construct load equal to the suture retention properties in human rotator cuff tendons after in vivo implantation at 12 weeks. Using reinforced fascia lata patches stitched with lyophilized human fascia lata from the iliotibial tract of human donors with 100% PLLA fibers in a canine infraspinatus model, Baker et al34 demonstrated greater ultimate load at time zero than with nonaugmented contralateral repairs. These parameters remained essentially unchanged at 12 weeks despite improvement in the ultimate load of nonaugmented controls.
Summary
In the literature, only Barber et al18 and Ciampi et al26 reported superior outcomes using GraftJacket and the polypropylene patch in the treatment of large to massive rotator cuff tears compared with conventional repairs. In our review, many of the studies of grafts that focus on human clinical outcomes rely on lower level case series or cohort studies. Therefore, further scientific development and associated randomized, controlled studies of biologic and synthetic grafts are required to determine the capability of these grafts to provide the mechanical and biologic properties necessary to restore function in patients with rotator cuff tears. Based on the available data, however, the use of augmentation grafts must be weighed against the associated costs of the graft material; the additional surgical time necessary for implantation; and the risks of inflammation, infection, and graft degradation.
References
Evidence-based Medicine: Levels of evidence are described in the table of contents. In this article, references 8 and 17 are level I studies. References 9 and 25 are level III studies. References 3, 11-15, 19-22, 24, and 26-28 are level IV studies.
References printed in bold type are those published within the past 5 years.
- Gomoll AH, Katz JN, Warner JJ, Millett PJ: Rotator cuff disorders: Recognition and management among patients with shoulder pain. Arthritis Rheum 2004;50(12): 3751-3761.
- Valentin JE, Badylak JS, McCabe GP, Badylak SF: Extracellular matrix bioscaffolds for orthopaedic applications: A comparative histologic study. J Bone Joint Surg Am 2006;88(12):2673-2686.
- Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K: The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am 2004;86(2):219-224.
- Rodeo SA: Biologic augmentation of rotator cuff tendon repair. J Shoulder Elbow Surg 2007;16(5 suppl):S191-S197.
- Weeks KD III, Dines JS, Rodeo SA, Bedi A: The basic science behind biologic augmentation of tendon-bone healing: A scientific review. Instr Course Lect 2014; 63:443-450.
- Adams JE, Zobitz ME, Reach JS Jr, An KN, Steinmann SP: Rotator cuff repair using an acellular dermal matrix graft: An in vivo study in a canine model. Arthroscopy 2006; 22(7):700-709.
- Derwin KA, Baker AR, Spragg RK, Leigh DR, Iannotti JP: Commercial extracellular matrix scaffolds for rotator cuff tendon repair: Biomechanical, biochemical, and cellular properties. J Bone Joint Surg Am 2006;88(12):2665-2672.
- Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ: Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears: A randomized, controlled trial. J Bone Joint Surg Am 2006;88(6): 1238-1244.
- Walton JR, Bowman NK, Khatib Y, Linklater J, Murrell GA: Restore orthobiologic implant: Not recommended for augmentation of rotator cuff repairs. J Bone Joint Surg Am 2007;89(4): 786-791.
- Gilbert TW, Freund JM, Badylak SF: Quantification of DNA in biologic scaffold materials. J Surg Res 2009;152(1):135-139.
- Cho CH, Lee SM, Lee YK, Shin HK: Mini- open suture bridge repair with porcine dermal patch augmentation for massive rotator cuff tear: Surgical technique and preliminary results. Clin Orthop Surg 2014;6(3):329-335.
- Badhe SP, Lawrence TM, Smith FD, Lunn PG: An assessment of porcine dermal xenograft as an augmentation graft in the treatment of extensive rotator cuff tears. J Shoulder Elbow Surg 2008;17(1 suppl):35S-39S.
- Sclamberg SG, Tibone JE, Itamura JM, Kasraeian S: Six-month magnetic resonance imaging follow-up of large and massive rotator cuff repairs reinforced with porcine small intestinal submucosa. J Shoulder Elbow Surg 2004;13(5):538-541.
- Soler JA, Gidwani S, Curtis MJ: Early complications from the use of porcine dermal collagen implants (Permacol) as bridging constructs in the repair of massive rotator cuff tears: A report of 4 cases. Acta Orthop Belg 2007;73(4):432-436.
- Gupta AK, Hug K, Boggess B, Gavigan M, Toth AP: Massive or 2-tendon rotator cuff tears in active patients with minimal glenohumeral arthritis: Clinical and radiographic outcomes of reconstruction using dermal tissue matrix xenograft. Am J Sports Med 2013;41(4):872-879.
- Arnoczky SP, Bishai SK, Schofield B, et al: Histologic evaluation of biopsy specimens obtained after rotator cuff repair augmented with a highly porous collagen implant. Arthroscopy 2016 Sep 17 [Epub ahead of print].
- Derwin KA, Codsi MJ, Milks RA, Baker AR, McCarron JA, Iannotti JP: Rotator cuff repair augmentation in a canine model with use of a woven poly-L-lactide device. J Bone Joint Surg Am 2009;91(5):1159-1171.
- Barber FA, Burns JP, Deutsch A, Labbé MR, Litchfield RB: A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy 2012;28(1):8-15.
- Zheng MH, Chen J, Kirilak Y, Willers C, Xu J, Wood D: Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA: Possible implications in human implantation. J Biomed Mater Res B Appl Biomater 2005;73(1):61-67.
- Gupta AK, Hug K, Berkoff DJ, et al: Dermal tissue allograft for the repair of massive irreparable rotator cuff tears. Am J Sports Med 2012;40(1):141-147.
- Bond JL, Dopirak RM, Higgins J, Burns J, Snyder SJ: Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: Technique and preliminary results. Arthroscopy 2008;24 (4):403-409.e1.
- Wong I, Burns J, Snyder S: Arthroscopic GraftJacket repair of rotator cuff tears.
J Shoulder Elbow Surg 2010;19(2 suppl): 104-109. - Agrawal V: Healing rates for challenging rotator cuff tears utilizing an acellular human dermal reinforcement graft. Int J Shoulder Surg 2012;6(2):36-44.
- McCormack RA, Shreve M, Strauss EJ: Biologic augmentation in rotator cuff repair: Should we do it, who should get it, and has it worked? Bull Hosp Jt Dis (2013) 2014;72(1):89-96.
- Proctor CS: Long-term successful arthroscopic repair of large and massive rotator cuff tears with a functional and degradable reinforcement device. J Shoulder Elbow Surg 2014;23(10):1508-1513.
- Ciampi P, Scotti C, Nonis A, et al: The benefit of synthetic versus biological patch augmentation in the repair of posterosuperior massive rotator cuff tears: A 3-year follow-up study. Am J Sports Med 2014;42(5):1169-1175.
- Encalada-Diaz I, Cole BJ, Macgillivray JD, et al: Rotator cuff repair augmentation using a novel polycarbonate polyurethane patch: Preliminary results at 12 months’ follow-up. J Shoulder Elbow Surg 2011;20 (5):788-794.
- Nada AN, Debnath UK, Robinson DA, Jordan C: Treatment of massive rotator-cuff tears with a polyester ligament (Dacron) augmentation: Clinical outcome. J Bone Joint Surg Br 2010;92(10):1397-1402.
- Audenaert E, Van Nuffel J, Schepens A, Verhelst M, Verdonk R: Reconstruction of massive rotator cuff lesions with a synthetic interposition graft: A prospective study of 41 patients. Knee Surg Sports Traumatol Arthrosc 2006;14(4):360-364.
- Hee CK, Dines JS, Dines DM, et al: Augmentation of a rotator cuff suture repair using rhPDGF-BB and a type I bovine collagen matrix in an ovine model. Am J Sports Med 2011;39(8): 1630-1639.
- Zhao S, Zhao J, Dong S, et al: Biological augmentation of rotator cuff repair using bFGF-loaded electrospun poly(lactide-co- glycolide) fibrous membranes. Int J Nanomedicine 2014;9:2373-2385.
- Yokoya S, Mochizuki Y, Natsu K, Omae H, Nagata Y, Ochi M: Rotator cuff regeneration using a bioabsorbable material with bone marrow-derived mesenchymal stem cells in a rabbit model. Am J Sports Med 2012;40(6):1259-1268.
- Aurora A, Mesiha M, Tan CD, et al: Mechanical characterization and biocompatibility of a novel reinforced fascia patch for rotator cuff repair. J Biomed Mater Res A 2011;99(2): 221-230.
- Baker AR, McCarron JA, Tan CD, Iannotti JP, Derwin KA: Does augmentation with a reinforced fascia patch improve rotator cuff repair outcomes? Clin Orthop Relat Res 2012;470(9):2513-2521.
From the Department of Orthopaedic Surgery, University Hospitals, Cleveland Medical Center
(Dr. Gillespie, Dr. Knapik, and Dr. Akkus), and the Department of Mechanical and Aerospace Engineering, Case Western Reserve University (Dr. Akkus), Cleveland, Ohio.
Dr. Gillespie or an immediate family member is a member of a speakers’ bureau or has made paid presentations on behalf of Wright Medical Technology and DJO Global. Neither of the following authors nor any immediate family member has received anything of value from or has stock or stock options held in a commercial company or institution related directly or indirectly to the subject of this article: Dr. Knapik and Dr. Akkus.
J Am Acad Orthop Surg 2016;24: 823-828
DOI: 10.5435/JAAOS-D-15-00229
Copyright 2016 by the American Academy of Orthopaedic Surgeons.


